Latest news
Media coverage, awards and more
We are thrilled to celebrate Aya Takeoka’s award of the JSPS Prize, Japan’s most distinguished recognition for early-career researchers. Each year, only 25 exceptional scholars are selected from the full spectrum of academic fields, spanning the humanities to the natural sciences. Being chosen among the small and extraordinarily talented group underscores the impact, creativity, and promise of Takeoka Lab’s research. This prestigious recognition reflects not only Aya’s scientific achievements but also her leadership, dedication to advancing knowledge, and commitment to fostering an environment where innovation thrives. The entire lab takes immense pride in this accomplishment! We congratulate Aya and look forward with excitement to the groundbreaking contributions that will undoubtedly follow.
Congratulations to Dr. Li-Feng Yeh on being awarded the highly prestigious Special Postdoctoral Researcher (SPDR) Fellowship from RIKEN! This honor recognizes exceptional scientific talent and is granted to only a select group of researchers each year. Li-Feng’s achievement reflects both outstanding research accomplishments and remarkable potential for future innovation. We look forward to celebrating continued success and the exciting discoveries that will emerge from Li-Feng’s future work!
Research Interests
“It is like riding a bike.” This phrase refers to memory retention of a learned task in the absence of practice. Our everyday experience shows that the nervous system forms long-term motor memories. However, experimental demonstration of how motor memories are formed and retained remains elusive.
Spinal circuit plasticity for motor control and learning
Spinal circuits are at the heart of sensorimotor transformation to generate movements. Yet, it is challenging to disentangle how spinal circuits might contribute to motor skill acquisition and retention due to the physical continuum of the brain that also participates in those processes. As such, we know little about the identities of spinal neurons or circuits that underlie the bottom-up mechanisms of motor skill learning and retention. Our recent work suggests the exciting possibility that specific cell types and circuits drive mechanisms regulating spinal learning and memory recall. We aim to understand circuit organization principles and functions that underlie motor adaptation and retention mediated by spinal circuits.
Motor control by sensory feedback
We are interested in understanding how sensory feedback regulates motor planning, learning, and execution. In this context, the lab focuses on dissecting how somatosensory, proprioceptive, and visual feedback is disseminated once the primary afferents enter the nervous system and are integrated to alter repetitive and complex motor behavior at the different hierarchical levels of the central nervous system.
A multidisciplinary approach
We use a wide variety of methods, including detailed motor kinematic assessments, mouse genetics, viral tracing and manipulation, in vitro and in vivo electrophysiological recordings in awake behaving animals, and imaging techniques. This combinatorial approach allows us to study and alter functions of specific neuronal populations, which in turn helps us to understand their roles in sensory information processing necessary for motor output and plasticity.
Publications
Main Publications
2024
Lavaud S, Bichara C, D’Andola M, Yeh S, Takeoka A.
Two inhibitory neuronal classes govern acquisition and recall of spinal sensorimotor adaptation.
Science 384,194-201.
2022
Bertels H, Vicente-Ortiz G, El Kanbi K, Takeoka A.
Neurotransmitter phenotype switching by spinal excitatory interneurons regulates locomotor recovery after spinal cord injury.
Nature Neuroscience 25 (5) 617–629.
2020
Takeoka A.
Proprioception: Bottom-up directive for motor recovery after spinal cord injury.
Neurosci Res 154, 1-8. Invited review.
2019
Takeoka A, Arber S.
Functional local proprioceptive feedback circuits initiate and sustain locomotor recovery after spinal cord injury.
Cell Reports 27 (1): 71–85.e3.
2016
Ruder L, Takeoka A, Arber S.
Long-distance descending spinal neurons ensure quadrupedal locomotor stability.
Neuron 92 (5): 1063-1078.
2015
Basaldella E, Takeoka A, Sigrist M, Arber S.
Multisensory signaling shapes vestibulo-motor circuit specificity.
Cell 163 (2): 301-12.
2014
Takeoka A, Vollenweider I, Courtine G, Arber S.
Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury.
Cell 159 (7): 1626-1639.
Preview article in the same issue
2011
Takeoka A, Jindrich DL, Muñoz-Quiles C, Zhong H, van den Brand R, Pham DL, Ziegler MD, Ramon-Cueto A, Roy RR, Edgerton VR, Phelps PE.
Axon regeneration can facilitate or suppress hindlimb function after Olfactory Ensheathing Glia transplantation.
J. Neurosci 31: 4298-4310.
Other publications
2023
Kolb J, Tsata V, John N, Kim K, Möckel C, Rosso G, Kurbel V, Parmar A, Sharma G, Karandasheva K, Abuhattum S, Lyraki O, Beck T, Müller P, Schlüßler R, Frischknecht R, Wehner A, Krombholz N, Steigenberger B, Beis D, Takeoka A, Blümcke I, Möllmert S, Singh K, Guck J, Kobow K, Wehner D.
Small leucine-rich proteoglycans inhibit CNS regeneration by modifying the structural and mechanical properties of the lesion environment.
Nat Commun 14, 6814.
2022
Rehman R, Miller M, Krishnamurthy S, Kjell J, Elsayed L, Hauck S, Heuvel F, Conquest A, Chandrasekar A, Ludolph A, Boeckers T, Mulaw M, Goetz M, Morganti-Kossmann M, Takeoka A, Roselli F.
Met/HGFR triggers detrimental reactive microglia in TBI.
Cell Reports 41, 111867.
2019
Ceyssens F, Carmona MB, Kil D, Deprez M, Tooten E, Nuttin B, Takeoka A, Balschun D, Kraft M, Puers R.
Chronic neural recording with probes of subcellular cross-section using 0.06 mm² dissolving microneedles as insertion device.
Sensors and Actuators B: Chemical 284: 369-376.
2011
Ziegler MD, Hsu D, Takeoka A,¬ Zhong H, Ramon-Cueto A, Phelps PE, Roy RR, Edgerton VR.
Further evidence of Olfactory Ensheathing Glia facilitating axonal regeneration after a complete spinal cord transection.
Exp Neurol 229: 109-119.
2010
Takeoka A, Kubasak MD, Zhong H, Kaplan JA, Roy RR, Phelps PE.
Noradrenergic innervation of the rat spinal cord caudal to a complete spinal cord transection: Effects of olfactory ensheathing glia.
Exp Neurol, 222, 59-69.
2009
Takeoka A, Kubasak MD, Zhong H, Roy RR, Phelps PE.
Serotonergic innervation of the caudal spinal stump in rats after complete spinal transection: effect of olfactory ensheathing glia.
J Comp Neurol, 515, 664-76.
2008
Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Muñoz-Quiles C, Roy RR, Edgerton VR, Ramón-Cueto A, Phelps PE.
OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats.
Brain, 131, 264-76.
Alumni
Former lab members
Postdocs
-
Charlotte Bichara
-
Jérémy Cousineau
-
Mattia D’Andola
-
Pierre-Arthur Suray
PhD Students
-
Hannah Bertels
-
Khadija El Kanbi
-
Simon Lavaud
-
Michael Aaron Miller
-
Guillem Vicente Ortiz
Master’s Students
-
Aditya Awasthi
-
Karen Bens
-
Noemi De Broeck
-
Arnaud Hallemans
-
Margo Hurlimans
-
Lauren Miguet
-
Haki Noori
-
Mathias Smeets
Technicians
-
Karin Jonckers
-
António José Folgado Laranjeira
Address
Laboratory for Motor Circuit Plasticity
RIKEN Center for Brain Science
Room 101b, CBS Neural Circuit Genetics Research Building
2-1 Hirosawa, Wako-shi,
Saitama 351-0198, Japan
Contact
Position Inquiries
Aya Takeoka - Team Leader
aya.takeoka (at) riken.jp
General Inquiries
Yuko Goto - Administrative Assistant
yuko.goto (at) riken.jp
+81-48-462-1111 ext. 6252 (from abroad)
048-462-1111 ext. 6252 (from Japan)
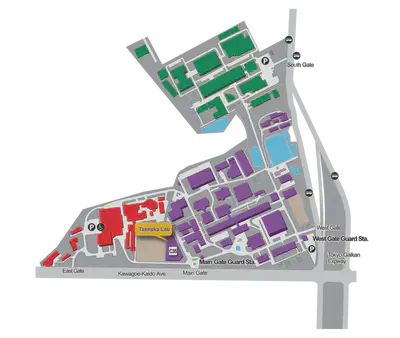
Directions
For directions to RIKEN please refer to this page.